Welcome to the Lung Cancer Biospecimen Resource Network (LCBRN).
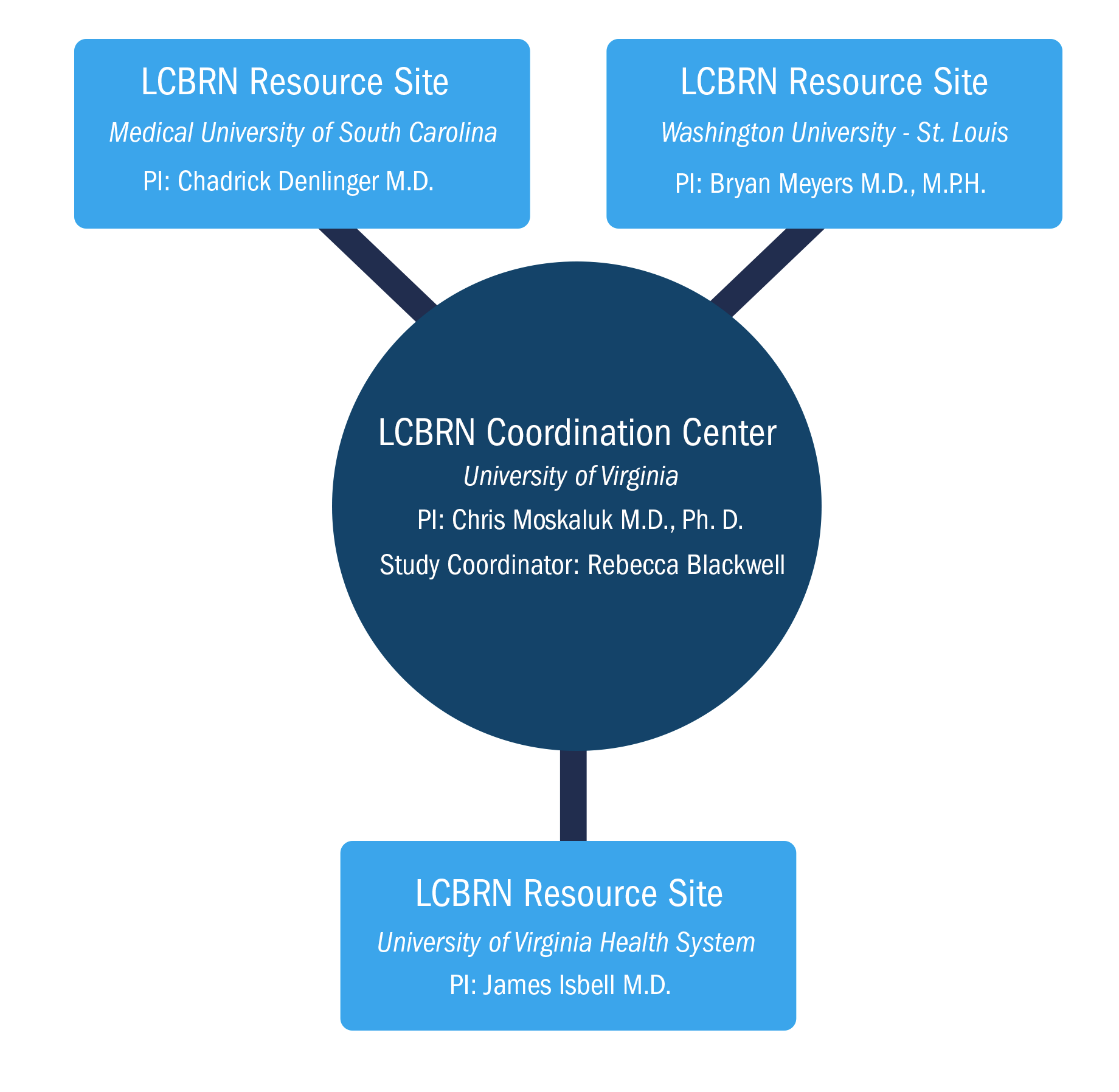
The LCBRN, a network of 3 academic medical centers, included the Medical University of South Carolina (MUSC), The University of Virginia (UVA) and Washington University in St. Louis (WUSTL). Biospecimens were collected at these sites according to standard operating procedures and were shipped to the LCBRN Coordination Center at UVA for storage. As an open access biorepository, the LCBRN provided specimens to academic and private industry scientists worldwide.

About LCBRN
The LCBRN obtained tissue and biofluid samples from lung cancer patients for use in biomedical research. Subjects voluntarily donated tissue, bronchial lavage fluid, blood, urine and saliva, and signed informed consent documents allowing the use of their samples in research.
The LCBRN, a network of 3 academic medical centers, included the Medical University of South Carolina (MUSC), The University of Virginia (UVA) and Washington University in St. Louis (WUSTL). Biospecimens were collected at these sites according to standard operating procedures and were shipped to the LCBRN Coordination Center at UVA for storage. As an open access biorepository, the LCBRN provided specimens to academic and private industry scientists worldwide.
Mission Statement
To collect, annotate, store, and distribute human lung cancer biospecimens in a manner that embraces the highest ethical standards for human subjects research, conforms to the best practices of biorepository science, and furthers basic, translational and clinical research in the understanding, diagnosis and treatment of this disease.
From May 2011 to August 2014, the LCBRN enrolled 763 patients, 78% of whom have contributed both lung tumor and baseline fluids. Follow-up data was collected every 6 months post baseline enrollment until August 2018. In September 2018, the LCBRN transferred all collections to the Cooperative Human Tissue Network, an NCI supported resource. All investigators interested in obtaining LCBRN samples after this time should contact the CHTN directly.
In the News