The LCBRN collects tissue and biofluids from consented patients undergoing surgical resection for known or suspected lung cancer. After a patient is consented for the LCBRN study, baseline fluids (saliva, serum, plasma, urine) are collected pre-operatively. The LCBRN also obtains baseline fluids from advanced stage lung cancer patients who are not candidates for resection but who require an operation for staging and/or diagnostic procedures.
| Fluid Types | Fluid Attributes |
|---|---|
|
Serum Plasma Buffy coat from plasma Urine Saliva Bronchial lavage Bronchial lavage cell pellet
|
No known cancer Pre-op, with cancer Post-op, no known cancer Post-op, with known cancer recurrence Late stage cancer, pre-treatment Late stage cancer, post-treatment
|
For subjects who are eligible for surgery, both bronchial washings and tissue are obtained intra-operatively. Tissue is procured following clinical assessment by a pathologist to determine if “left over” remnant tissue is available for research procurement.
| Tissue Types Available | TMAs Available |
|---|---|
|
Malignant lung Non-neoplastic lung Non-neoplastic bronchus Non-neoplastic lung from non-cancer patients |
| DNA, RNA and Protein Extracts* Available |
|---|
|
Malignant lung Non-neoplastic lung |
*DNA and RNA are available as 10 uL aliquots of 100-150 ng/uL concentration. The RNA isolation procedures obtain low molecular weight (miRNA, etc. – see figure below) as well as high molecular weight species. Protein extracts are split TCA precipitates from approximately 100 mg tissue samples. See SOP #14 and SOP#15.
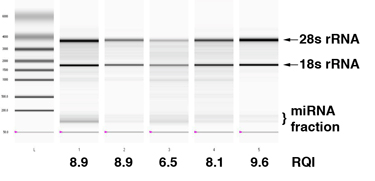
Example of microcapillary electrophoresis of 5 tumor RNA samples, isolated using the LCBRN protocol. An RQI index of 6 is the lower cutoff for acceptable samples, but samples with lower scores may be made available to investigators for whom RNA quality is not an issue.
After surgery, LCBRN subjects are followed up at sequential 6-month intervals during which biofluids are again obtained. Clinical data is still obtained from any subjects who are unable to return for follow-up specimen collection.
| Summary of Procurement Events |
|---|
|
Baseline collection (pre-op): serum, plasma, urine, saliva Surgical procedure (intra-op): tissue, bronchial lavage fluid 1st follow-up visit (post-op): serum, plasma, urine, saliva Subsequent follow-up visits (post-op): serum, plasma, urine |
