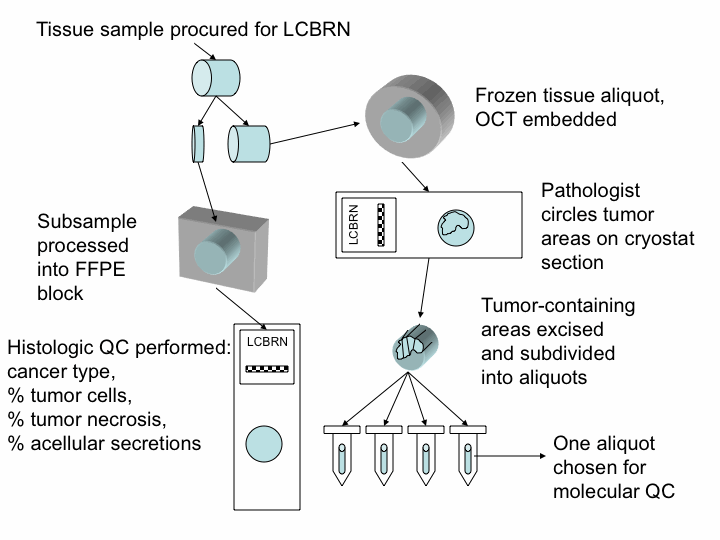
The subjects who have volunteered to provide samples to the LCBRN have undergone surgical procedures to remove lung cancers as part of their routine clinical care. All tissue samples obtained for the LCBRN are remnant (excess) tissue from these surgical procedures, which were not required for clinical staging and diagnostic procedures. The standard operating procedure used in procuring, documentation and quality control of tissue specimens can be obtained on our SOPs page.
All tissue samples obtained for the LCBRN are subdivided so that a portion is formalin fixed and processed for routine histologic assessment. The remaining tissue is snap frozen. All frozen tissue is embedded in OCT compound and a frozen histologic section is taken for microscopic assessment. For tumor samples, areas that contain the highest concentration of viable tumor are subdivided into smaller segments (approximately 100 mg) for distribution. The approximate tumor cellularity and percent tissue necrosis are estimated and recorded. Samples of non-neoplastic lung and non-neoplastic bronchus are similarly procured and analyzed histologically.
For most cases a segment of tumor and a segment of non-neoplastic lung tissue are processed for simultaneous DNA, RNA and protein extraction. Aliquots of these molecular fractions are also available to investigators. The tumor RNA is analyzed by microcapillary electrophoresis (Experion, BioRad), which serves as the quality control metric for the molecular integrity of the specimen. An RNA Quality Index (RQI) score is then determined.